Sarcoidosis is a complex disease that affects multiple species. The development of granulomatous lesions occurring in one or multiple organ systems is characteristic for this disorder and has been described in human, bovine and equine medicine (Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Ungprasert et al, 2019; Ilha et al, 2023). The number of case reports in horses has gradually increased over time, which could suggest that sarcoidosis is not as rare as previously assumed (Rose et al, 1998; Reijerkerk et al, 2008; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Schwarz, 2022). Little is known about the pathophysiology, although an exaggerated immune response to one or more unknown triggers is suspected to be the origin. In horses, recognition and definitive diagnosis of sarcoidosis may be challenging as the clinical picture varies greatly. Equine sarcoidosis reportedly affects various organ systems, but granulomatous inflammation occurs consistently in the skin in most cases described in the literature. Equine sarcoidosis is diagnosed by exclusion of differential diagnoses that cause a similar clinical or histological picture. To date, specific diagnostic tests are lacking. Unfortunately, treatment with immunosuppressors is rarely curative and for some forms of the disease, the prognosis for life is poor (Reijerkerk et al, 2009; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b; Rashmir-Raven, 2018). This article provides an overview of the clinical picture, diagnosis and treatment of sarcoidosis in horses.
Forms of equine sarcoidosis
Three disease phenotypes have been described in horses: localised, partly generalised and generalised (Reijerkerk et al, 2009; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). Looking at larger case studies in the literature, the localised form seems to be most frequently diagnosed (Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Schwarz, 2022). Unfortunately, the current data are quantitatively insufficient to draw relevant conclusions regarding the incidence of this disease (Wimmer-Scherr and Schwarz, 2025). The localised phenotype affects a limited area of the skin, most commonly the lower limbs, in otherwise systemically healthy animals (Reijerkerk et al, 2009; Scott and Miller, 2010; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). In the partly generalised form, multiple and larger areas of the skin are involved and peripheral lymphadenopathy occurs. This form often deteriorates into the generalised form with lesions spreading over multiple organ systems (Sargent et al, 2007; Scott and Miller, 2010; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b; Rashmir-Raven, 2018). The generalised form (idiopathic systemic granulomatous disease) leads to various systemic clinical signs dependent on the distribution of granulomatous lesions in the body (Sargent et al, 2007; Scott and Miller, 2010; Rashmir-Raven, 2018). The skin, lymph nodes and lungs are most commonly affected, but lesions have been described in almost every organ, including the gastrointestinal tract, liver, spleen, kidneys, adrenal glands, bones, heart, thymus and central nervous system (Axon et al, 2004; Spiegel et al, 2006; Hansmann et al, 2007; Reijerkerk et al, 2008; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Nolte et al, 2020).
Clinical signs
Although suspected by some researchers, no sex or breed predilection for equine sarcoidosis has been proven at the time of writing (Rose et al, 1998; Spiegel et al, 2006; Reijerkerk et al, 2008; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Schwarz, 2022). The disease can occur at any age, although most affected horses are older than 3 years of age (Loewenstein et al, 2004; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013b; Schwarz, 2022). The timeline of disease onset and progression can be highly variable, but sarcoidosis generally progresses slowly (Sargent et al, 2007). Depending on the location of granulomatous lesions in the body, sarcoidosis presents with various clinical manifestations and different outcomes (Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b).
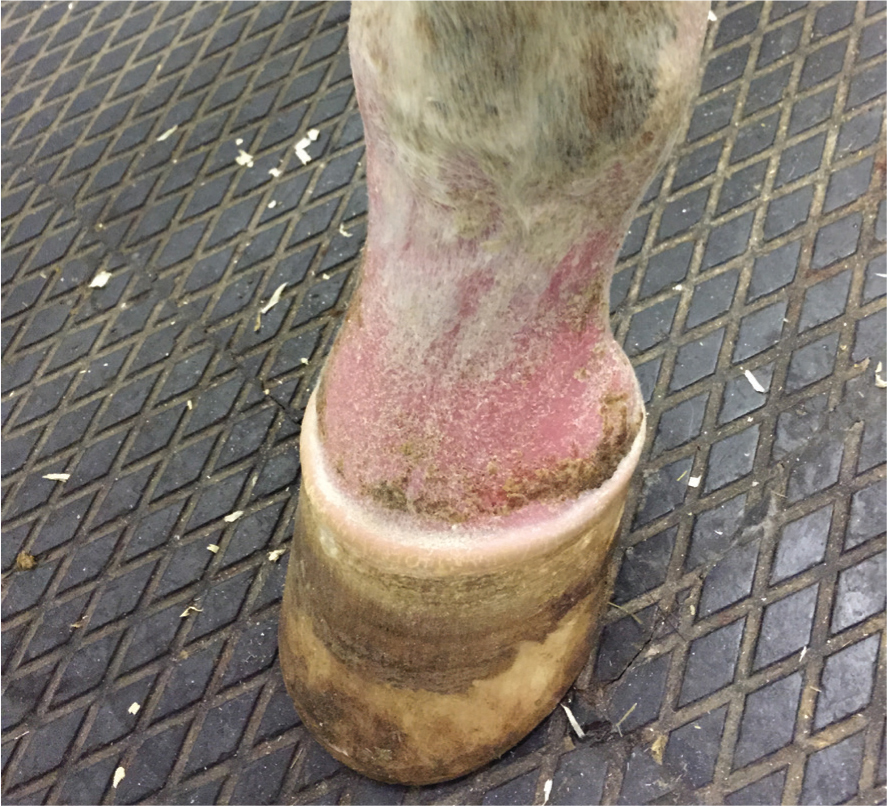
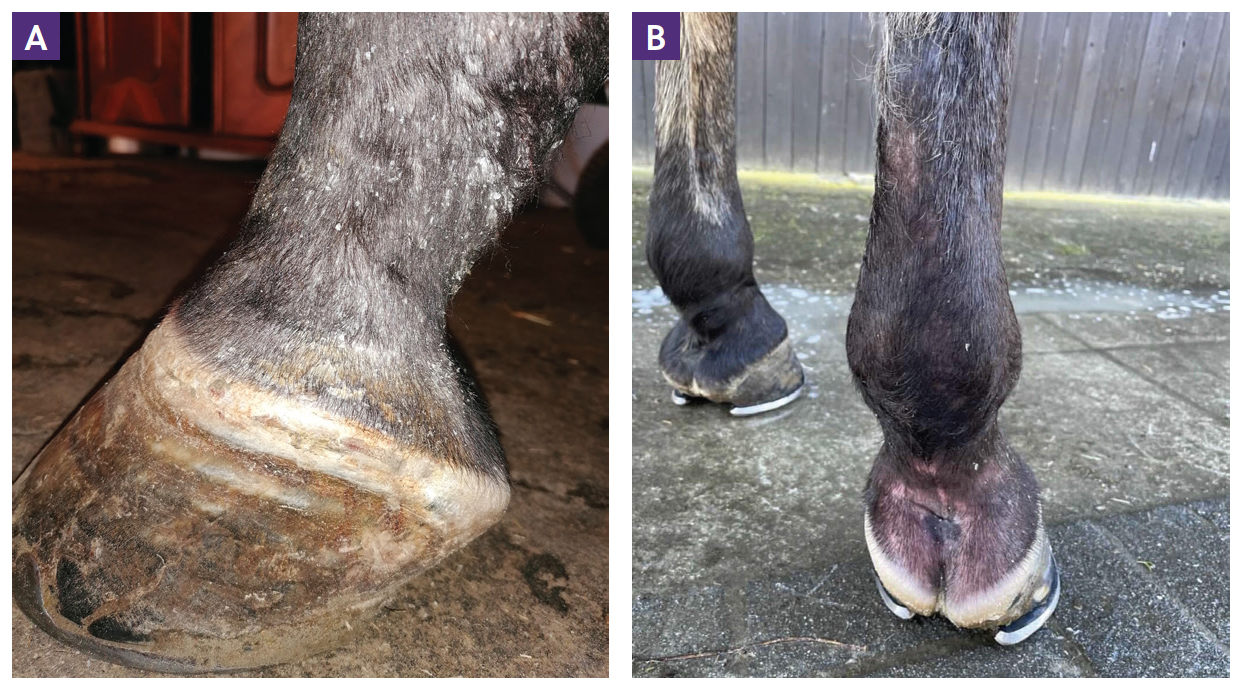
Dermatitis is the most consistent clinical sign among all forms of sarcoidosis. However, generalised cases without initial skin involvement have been reported on rare occasion (Axon et al, 2004). In general, two types of skin lesions have been described: scaling and crusting or, less commonly, nodular lesions. Both types of lesions may occur concurrently (Stannard, 1987; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). Characteristic demarcated areas of hyperkeratosis and crusting are often accompanied by variable degrees of alopecia (Figure 1). In addition, affected areas may appear warm, oedematous and sensitive to touch (Spiegel et al, 2006; Hansmann et al, 2007; Reijerkerk et al, 2009; Schwarz, 2022). Occasionally, pruritus is also seen (Spiegel et al, 2006). The skin of the lower limbs is a classical site for sarcoidosis lesions, especially in the localised form, although lesions have been documented in numerous locations all over the body surface (Figure 2). The mane and tail seem to be rarely affected (Rose et al, 1998; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). Pain originating from dermatitis on the legs can result in lameness (Scott and Miller, 2010; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a). Laminitis might be a consequence of coronary band involvement in equine sarcoidosis (Schwarz, 2022) (Figure 3a; 3b).

The generalised form of sarcoidosis is characterised by nodular and tumour-like masses in multiple organ systems (Axon et al, 2004; Reijerkerk et al, 2008; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). The so-called ‘wasting syndrome’ that results from granulomatous inflammation spreading throughout the body typically manifests in nonspecific systemic clinical signs (Reijerkerk et al, 2009; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). Anorexia and weight loss, exercise intolerance, depression and fever can appear alongside other symptoms specific to the organ systems affected. In the literature, some horses with sarcoidosis were also reported to show ataxia, diarrhoea, ventral oedema and/or respiratory signs like coughing and mild dyspnoea (Heath et al, 1990; Axon et al, 2004; Spiegel et al, 2006; Reijerkerk et al, 2008; Scott and Miller, 2010; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013b; Kutasi et al, 2014; Nolte et al, 2020).
Pathogenesis
To date, the cause of equine sarcoidosis remains unknown. While the basic pathophysiological mechanism is suspected to be an exaggerated immune response to one or more antigenic triggers, further research is required to validate this hypothesis (Reijerkerk et al, 2009; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b; Rashmir-Raven, 2018). Possible aetiological agents that have been investigated in equine medicine include different bacteria, equine herpesvirus and hairy vetch poisoning, but results were either negative or contradictory (Woods et al, 1992; Spiegel et al, 2006; White et al, 2009; Oliveira-Filho et al, 2012; Nolte et al, 2020). Polymerase chain reaction analysis of the affected tissues of eight cases of equine sarcoidosis was negative for mycobacterial, coccidial, cryptococcal, corynebacterial and Borrelia burgdorferi DNA (Spiegel et al, 2006). In addition, no equine herpesvirus-1 or -2 DNA was detected upon further examination of those samples in a subsequent study (White et al, 2009). Different histological staining methods of the same samples did not provide relevant findings (Spiegel et al, 2006; White et al, 2009). Contradictory results were published by Oliveira-Filho et al (2012), who detected mycobacterial DNA in affected tissues of one case of generalised sarcoidosis. Similarly, Nolte et al (2020) found equine herpesvirus-2 DNA in affected brain and skin tissue, contrary to previous findings. Hairy vetch (Vicia villosa) poisoning has also been discussed as a possible aetiologic agent for sarcoidosis in cows and horses (Woods et al, 1992; Ilha et al, 2023). More research is needed to determine the exact cause of this disease.
Diagnosis
No specific diagnostic test for equine sarcoidosis has been established to date. Definitive diagnosis of this disease is based on a synergy of multiple factors: a compatible anamnesis, exclusion of other differential diagnoses and evidence of granulomatous inflammation in affected tissues (Sargent et al, 2007; Reijerkerk et al, 2009; Scott and Miller, 2010; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). Similar skin lesions to those occurring with sarcoidosis may be caused by other diseases like dermatophytosis, dermatophilosis, bacterial folliculitis and furunculosis, insect bite hypersensitivity, multisystemic eosinophilic epitheliotropic disease, erythema multiforme, pemphigus foliaceus, neoplasia and ectoparasites. In addition, drug interactions or toxicoses caused by arsenic, iodine or vetch should be excluded (Stannard, 1987; Rose et al, 1998; Stannard, 2000; van Oldruitenborgh-Oosterbaan and Goehring, 2001; Spiegel et al, 2006; Sargent et al, 2007; Reijerkerk et al, 2009; Scott and Miller, 2010; Paterson and Ball, 2013; Rashmir-Raven, 2018).
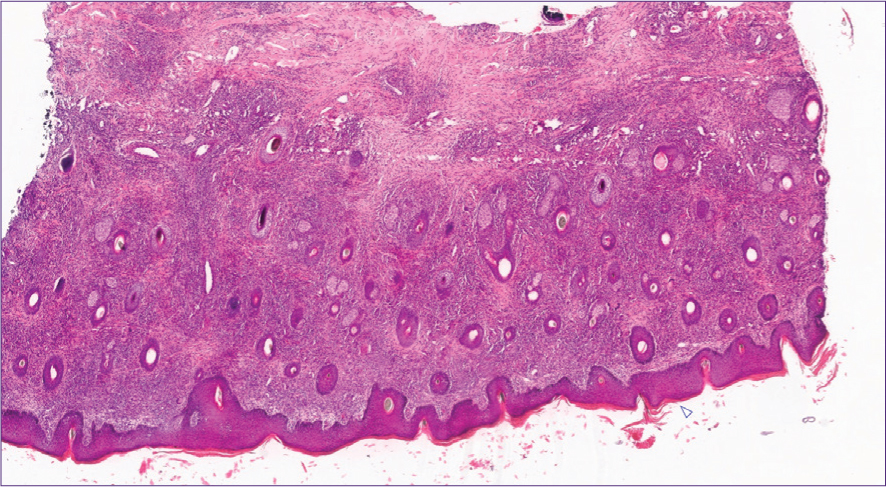
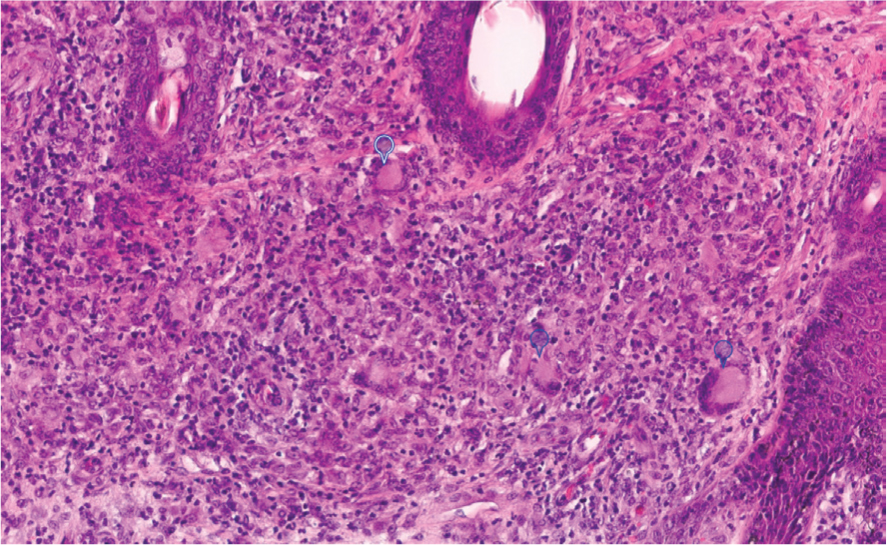
With equine sarcoidosis, histological examination of skin biopsies typically reveals deep infiltration with multinucleated giant cells alongside macrophages, lymphocytes and plasma cells (Figure 4). Vasculitis and infiltration with eosinophiles may or may not accompany the histopathological picture. Hyperkeratosis, hyperplasia (Figure 4a) and sometimes neutrophilic infiltration of the overlying epidermis may be evident. Nodular lesions usually contain compact clusters of epithelioid cells with multinucleated giant cells and sometimes central necrosis (Sellers et al, 2001; Hansmann et al, 2007; Reijerkerk et al, 2009; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Schwarz, 2022). Granulation tissue formation may occur in some advanced cases (Hansmann et al, 2007; Nolte et al, 2020).
The clinical picture can help to differentiate between the three forms of sarcoidosis (Sargent et al, 2007; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). However, evidence of granulomatous lesions in organs other than the skin may be obtained for definitive diagnosis of the partly generalised and generalised form of equine sarcoidosis. This can be achieved via medical imaging of internal organs and histopathology of suspicious tissues (Sargent et al, 2007). Equine sarcoidosis produces unspecific changes in different diagnostic imaging techniques, eg interstitial lung pattern in radiographic images of the thorax if the lungs are affected. Futhermore, multiple different organs may be affected by the disease. Diagnostic imaging in this context is helpful to point out which organs may be affected by the disease and to help identify suitable locations for biopsies to ultimately confirm the diagnosis (Wimmer-Scherr and Schwarz, 2025), but a full discussion of these is beyond the scope of this manuscript. As the lymph node and lungs are the most commonly affected internal organ systems in generalised cases of sarcoidosis, they should be prioritised in the diagnostic workup (Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b). More specific symptoms pointing to other organ systems may guide the clinician in their choice of specific diagnostic tests. A complete blood count and serum chemistry profile commonly only reveal unspecific signs of chronic inflammation in horses with sarcoidosis but may be useful to exclude differential diagnoses (Rose et al, 1998; Spiegel et al, 2006; Sargent et al, 2007; Reijerkerk et al, 2008; Kutasi et al, 2014).
Treatment
The current treatment options for sarcoidosis in equine and human medicine are the administration of different immunosuppressant agents to stop further granuloma formation (Sargent et al, 2007; Reijerkerk et al, 2009; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b; Ungprasert et al, 2019). The use of dexamethasone, prednisolone and methotrexate against sarcoidosis has been documented in the literature (Heath et al, 1990; Rose et al, 1998; Spiegel et al, 2006; Sargent et al, 2007; Reijerkerk et al, 2008; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Nolte et al, 2020; Schwarz, 2022). However, because of low case numbers in the literature and a complete lack of prospective treatment studies, there is mostly anecdotal evidence of the efficacy of those drugs in equine sarcoidosis. In addition, affected horses frequently receive a multitude of different medications including antibiotics and topical agents concurrently, which makes it difficult to judge the effectiveness of individual drugs (Spiegel et al, 2006; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Schwarz, 2022).
High doses of corticosteroids are suggested for initial treatment of equine sarcoidosis. Depending on the response to therapy, doses should be gradually tapered to a minimum effective dose for continuous long-term treatment. Suggested initial doses range between either 1–4 mg/kg for oral prednisolone or 0.04–0.2 mg/kg for intramuscular dexamethasone (Reijerkerk et al, 2009; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b; Rashmir-Raven, 2018). Some authors advocate for initial dosing at the higher end of the suggested dosing interval to achieve immunosuppression rather than only anti-inflammatory treatment (Scott and Miller, 2010). However, the side effects of corticosteroid use should be taken into account when establishing a treatment plan in each individual horse (Reijerkerk et al, 2009).
In case of treatment failure or contraindications to corticosteroid use, methotrexate can serve as a second line agent in the treatment of sarcoidosis (personal communication with Derek Knottenbelt in 2020). This folate antagonist seems to prove effectiveness in the treatment of some cases of immune-mediated disease in horses and donkeys (Mosca et al, 2020; Schwarz, 2022). According to Rostang et al (2020), 0.2 mg/kg methotrexate subcutaneously once a week is a safe and effective dose for long-term treatment in healthy horses. Because of the lack of prospective clinical studies investigating the use of methotrexate in diseased animals, the dosing regimen established by Rostang et al (2020) can serve as a basis for the development of a treatment plan in sarcoidosis. Alternatively, oral treatment at a dose of 0.25 mg/kg methotrexate every 72 hours for three consecutive doses, followed by dosing at increased intervals and subsequent maintenance therapy to effect, has been successfully applied in some cases of sarcoidosis (personal communication with Derek Knottenbelt in 2021).
Many different topical agents have been suggested for the treatment of skin lesions in equine sarcoidosis, but none of those treatments are evidence-based. Topical treatment should be completely avoided when skin lesions are painful to touch (Reijerkerk et al, 2009; Sloet Van Oldruitenborgh-Oosterbaan and Grinwis, 2013b).
In cases of equine sarcoidosis, stopping or delaying disease progression is counted as treatment success in the literature as existing granulomatous lesions usually persist despite treatment. Furthermore, long-term administration of medication is frequently needed to maintain the achieved treatment success (Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Schwarz, 2022). Documented response to treatment is highly variable but tends to be better for localised cases of sarcoidosis than for the partly generalised and generalised forms. Generally, it is difficult to objectively judge treatment response as skin lesions tend to wax and wane without linear improvement. Comparison among studies that retrospectively document the effectiveness of different treatment methods is rather challenging (Rose et al, 1998; Spiegel et al, 2006; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Schwarz, 2022).
It is important to state that none of the treatment protocols discussed in this article are based on strong evidence. However, because of the lack of sufficient research, existing anecdotal evidence may serve as a basis in the establishment of treatment protocols in individual cases of equine sarcoidosis.
Prognosis
Prognosis for survival is generally good for the localised form of sarcoidosis, although remission of skin lesions is uncommon. However, complete remission with and without treatment has been reported in the literature. In case of coronary band involvement, possible development of laminitis may worsen the prognosis for survival (Schwarz, 2022). As the partly generalised and generalised form of equine sarcoidosis frequently show insufficient response to therapy, affected animals are often euthanised on humane grounds soon after diagnosis of the condition or as the disease progresses (Axon et al, 2004; Spiegel et al, 2006; Hansmann et al, 2007; Reijerkerk et al, 2008; Oliveira-Filho et al, 2012; Sloet van Oldruitenborgh-Oosterbaan and Grinwis, 2013a; Kutasi et al, 2014; Schwarz, 2022).
Conclusions
Sarcoidosis remains a poorly understood disorder in horses. Treatment attempts are often unsuccessful or provide inconsistent results, which is frustrating for horse owners and veterinarians. Some clinical manifestations of this disease have devastating effects on the welfare of the animal and euthanasia is not uncommon. Further detailed retrospective case reports may help to provide a basis for targeted research into the pathophysiology of equine sarcoidosis. Prospective, double-blinded clinical studies are needed to investigate the effects of different treatment protocols in the management of this disease. Multicentre studies might be an appropriate setting to gather a sufficient number of cases of this rare disease to boost statistical significance of study results. Furthermore, this might ultimately help to clarify if equine sarcoidosis is as rare as assumed in earlier studies or is simply an under-recognised disease.


