Mammalian kidneys have many essential functions to maintain homeostasis. Two of their primary functions are the excreting the waste products of metabolism and exogenous chemicals and controlling the volume and composition of body fluids. Multiple other renal processes serve to affect these primary functions, including the regulation of fluid osmolality, regulation of the acid–base balance, regulation of arterial blood pressure, production, metabolism and excretion of certain hormones, and gluconeogenesis. These functions are so integral that after total loss of renal function, death of most mammals occurs within one week (Finco, 2008).
Renal functional anatomy
The functional unit of the kidney is the nephron (Figure 1). Each equine kidney contains close to 10 million nephrons, which do not increase in number after birth (Beech et al, 2001). Nephrons can be classified as cortical or juxtaglomerular, and cannot be regenerated; thus, this number decreases with age and disease. Cortical nephrons are located in the cortex, closer to the renal capsule, and extend for a shorter distance into the medulla than juxtaglomerular nephrons. Cortical nephrons are primarily responsible for the filtration of plasma, whereas juxtaglomerular nephrons play more of a role in urine concentration. In humans, approximately 75% of nephrons are cortical but the distribution is unknown in horses (Hall, 2016). Each nephron is composed of a renal corpuscle, a proximal tubule, a loop of Henle, a distal convoluted tubule, a connecting tubule and collecting ducts. The renal corpuscle is composed of the glomerulus surrounded by Bowman's capsule. Blood flows into the glomerular capillaries where fluid is filtered under high hydrostatic pressures into Bowman's space. As the filtrate flows through the sequence of tubules, it is modified through electrolyte, water and acid–base absorption and secretion. The resultant solution is urine that flows from the collecting ducts of the kidney into the renal pelvis, ureters and bladder, and is excreted from the body.
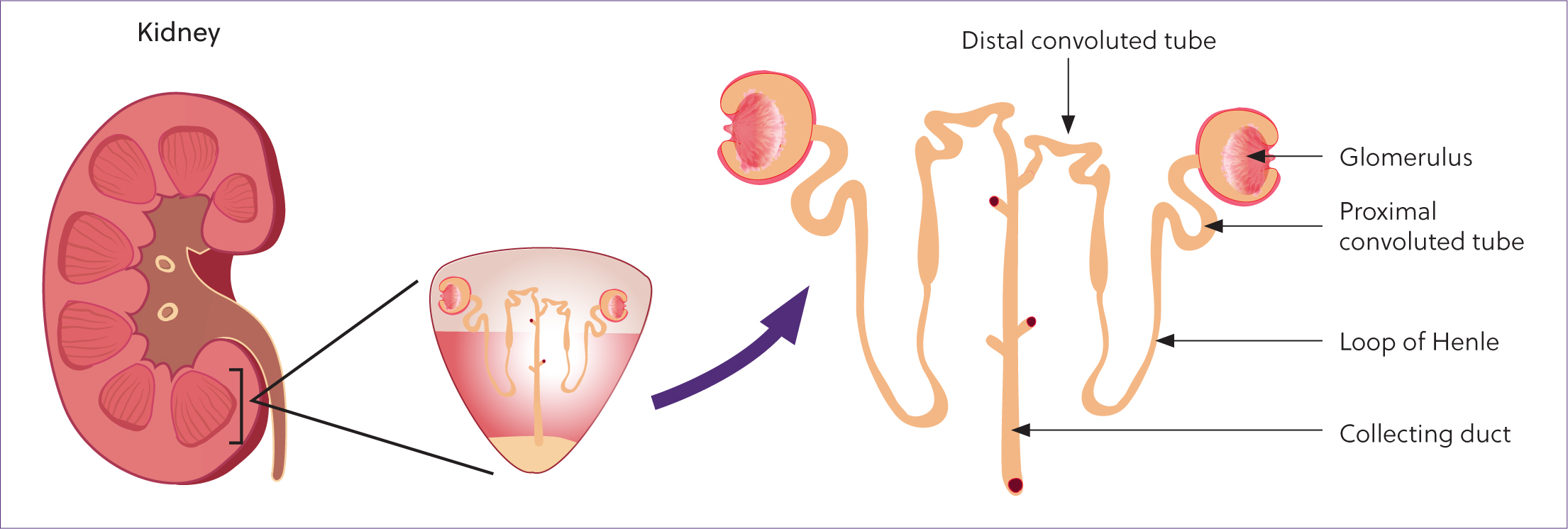
Glomerular filtration
Urine is formed through the combination of glomerular filtration, reabsorption of substances from the renal tubules and secretion of substances into the tubules. The composition of glomerular filtrate is almost exactly that of blood, without the presence of cells and most proteins. The glomerular filtration rate is determined by the net filtration pressure (hydrostatic and colloid osmotic pressure across the filtration membrane) and the glomerular capillary filtration coefficient. The glomerular capillary filtration coefficient is determined by the surface area of the glomerular membrane and its permeability. Under normal conditions, glomerular filtration rate is regulated through physiological control of glomerular capillary hydrostatic pressure and glomerular capillary colloid oncotic pressure. Glomerular capillary plasma hydrostatic pressure is determined by (Hall, 2016):
Regulation of glomerular filtration rate is mediated by the sympathetic nervous system, hormonal control of renal circulation, locally acting vasoactive substances and local feedback mechanisms. Autoregulation of glomerular filtration rate and renal blood flow maintains these parameters as constants in the face of changes in arterial blood pressure.
Renal tubular function
Glomerular filtrate passes into the renal tubules. Throughout this passage, selective reabsorption of substances from the filtrate into the blood, and secretion of substances from the blood into the filtrate occur, resulting in the production of urine (Figure 2). Thus, urine production is dependent upon three major processes: glomerular filtration, tubular reabsorption and tubular secretion.
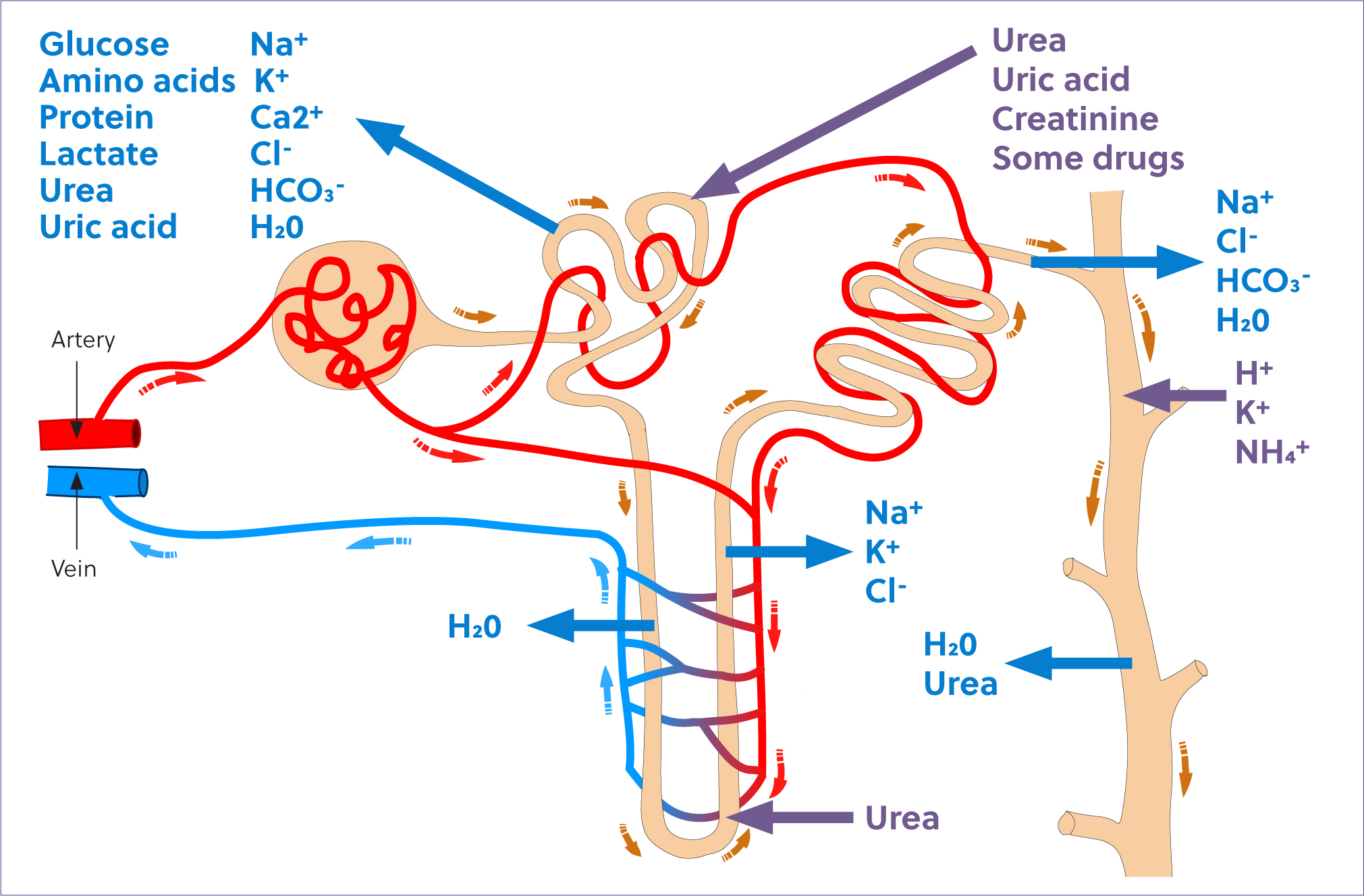
Tubular reabsorption occurs to a greater extent than tubular secretion. Tubular reabsorption is also highly selective and closely regulated to control the rate of conservation or excretion of substances, independent of each other. This precise and individual control allows for regulation of the composition of body fluids, one of the major functions of the renal system. The proximal tubule is responsible for reabsorption of nearly 100% of glucose and amino acids in the filtrate, around 90% of bicarbonate and a net of between 60% and 80% of sodium, chloride and water (Rose, 1989). The proximal tubules are also responsible for secretion of urea, ammonium ions and some other substances. The majority of ion reabsorption is the result of active transport, and the proximal tubule epithelial cells have a high metabolic rate. Despite having a relatively higher degree of perfusion than the medullary components of the nephron, the proximal tubules are highly susceptible to hypoxic injury when renal blood flow is reduced.
As tubular fluid passes through the descending loop of Henle, it becomes progressively more hypertonic as a result of reabsorption of water through the permeable tubular epithelium. The epithelium of the ascending loop of Henle is relatively impermeable to water but actively reabsorbs sodium, chloride and potassium, thus reducing the tonicity of the tubular filtrate (Rose, 1989). The active reabsorption of these ions is the basis of the countercurrent multiplication mechanism that facilitates water absorption and therefore, the ability to concentrate urine.
The distal tubule is responsible for additional minor modification of the composition of urine. It is an important site of calcium, potassium and acid secretion. Tubule fluid passes from the distal tubule into the collecting ducts, where further water reabsorption occurs and the final composition of urine is achieved.
Close proximity of the vascular system is integral to the function of the renal tubules. Substances reabsorbed from the tubules are easily transferred into the vasculature and help to create concentration gradients that favour reabsorption, and to maintain plasma osmolality. The vasa recta are capillaries that parallel the loops of Henle. Blood flow through the vasa recta is slow, and facilitates creation and maintenance of the countercurrent exchange system. The renal cortex is more highly vascularised than the medulla, with the medulla existing in a normally hypoxic environment (Schott et al, 2018). While the cortically located proximal tubules are highly susceptible to hypoxic injury because of their high metabolic rate, medullary injury predominates during times of renal hypoxia as a result of the exacerbation of this normal medullary hypoxia. Thus, injury to the distal tubules may be of more significance during times of renal hypoperfusion (Schott et al, 2018).
The equine tubular system is unique in its excretion of large amounts of calcium and mucus in the urine. Mucus is secreted by glands in the renal pelvis and proximal ureter, and helps to prevent mucosal injury because of the high concentration of calcium carbonate crystals in equine urine. The differences in calcium excretion in horses as compared to other species is not completely understood, but differences in the role of vitamin D in calcium and phosphorus maintenance have been identified and are likely involved (Breidenbach et al, 1998).
Assessing renal function
Renal dysfunction in horses may occur secondary to changes in haemodynamics, intrinsic renal disease or post-renal disease. The most common clinical signs associated with acute or chronic kidney disease are non-specific and, in cases of acute kidney injury, often secondary to concurrent disease processes. The most common clinical sign of chronic kidney disease in horses is weight loss, followed by polyuria/polydipsia, and ventral oedema (Schott et al, 1997). Other signs include poor performance, inappetance and unthrifty hair coat. Some horses with chronic kidney disease may also display signs of oral and upper gastrointestinal tract disease, including increased dental tartar accumulation, gingivitis, or oral or upper gastrointestinal tract ulceration.
Acute and chronic kidney disease in horses have not been extensively studied, and reports in literature likely do not reflect their prevalence in clinical practice. One early retrospective study in the United States found a prevalence of chronic kidney disease of 0.12% over a 32-year study period (Schott et al, 1997). A more recent study of acute kidney injury in horses found a prevalence of 14.8% in horses presenting to a referral facility (Savage et al, 2019). Causes of acute kidney injury in horses are common and include haemodynamic aetiologies resulting in renal hypoxia (sepsis, perinatal asphyxia syndrome, hypotension, disseminated intravascular coagulation), nephrotoxic drug administration (non-steroidal anti-inflammatory drugs, aminoglycosides, oxytetracycline), and myopathy or haemolysis (Divers, 2022). Accurate determination of renal function is useful for the prevention and early detection of kidney disease in horses, and is essential for monitoring disease progression or any response to treatment.
Glomerular filtration rate is the most useful measure of renal function and can be directly estimated by the serum clearance of a biomarker. Ideal markers of glomerular filtration rate should be freely filtered by the kidneys, without tubular secretion or reabsorption and should not be protein-bound or metabolised before filtration. Clearance of multiple substances (inulin, radionuclides, iohexol) have been evaluated as markers of glomerular filtration rate in horses (Matthews et al, 1992; Gonda et al, 2003; Wilson et al, 2009; Meucci et al, 2015). Glomerular filtration rate can also be indirectly measured by serum or plasma concentrations of endogenous biomarkers (creatinine, serum urea nitrogen, symmetric dimethylarginine), with an increase in concentration reflecting a decrease in the glomerular filtration of that marker. In humans, estimated glomerular filtration rate is determined based on prediction equations based on measured creatinine, urea and albumin, as well as age, gender and race (Miller et al, 2018). Estimated glomerular filtration rate has not been developed for use in horses, and the large variability in equine body size and muscle mass would make derivation of an accurate equation difficult.
Creatinine and serum urea nitrogen
Serum urea nitrogen and serum creatinine concentrations are the most commonly used indices of renal function as measurements of renal retention of nitrogenous wastes. Serum urea nitrogen and creatinine are included in most blood biochemistry profiles, and are readily clinically available and inexpensive assays to perform. Because of the extensive renal reserve capacity, changes in serum urea nitrogen and creatinine do not occur until approximately 75% of glomerular filtration rate has been affected (Schott et al, 2018). As such, changes in serum urea nitrogen or creatinine are insensitive indicators of early or minor changes in renal function. However, small changes in serum urea nitrogen or creatinine, even while remaining within reference intervals, reflect significant changes in glomerular filtration rate. Therefore, monitoring changes in creatinine over time, rather than absolute value, is useful in detecting early acute kidney injury.
Creatinine is a normal byproduct of hydrolysis of creatine phosphate in muscle. Creatinine is produced at a constant rate in the normal animal and freely filtered by the glomerulus without tubular reabsorption, meaning plasma creatinine concentration can serve as an indirect measurement of glomerular filtration rate. Creatinine can be affected by non-renal factors resulting in increases (fasting, rhadomyolysis, exercise) or decreases (muscle wasting, emaciation) in measured plasma concentrations (Schott et al, 2018). On the other hand, when serum bilirubin increases above 5 mg/dL, measured creatinine may be falsely reduced by 0.1–0.5 mg/dL (Brenner, 2008).
The most frequently used method of determining plasma creatinine concentration is the Jaffe method, a colorimetric assay based on the formation of a complex between creatinine and alkaline picrate (Finco, 2008). Non-creatinine chromagens (glucose, pyruvate, acetoacetate, fructose, uric acid and ascorbic acid) in the serum are also detected by the Jaffe method and may result in overestimation of the true creatinine concentration. As serum creatinine concentrations increase, the proportion of non-creatinine chromagens decreases and the total measured value becomes more accurate. Thus, increases in plasma creatinine concentration are more accurate and sensitive for patients with severe renal dysfunction than in early or mild cases.
Urea nitrogen is produced by the liver after ammonia uptake and metabolism. The amount of ammonia taken up by the liver is dependent upon dietary protein and amino acid intake, the amount of amino acids and proteins that are broken down to ammonia and the rate of catabolism of lean body tissue. An increase in serum urea nitrogen may reflect increased protein catabolism rather than decreased urinary excretion (Finco, 2008). In humans and other species, processes that increase protein catabolism resulting in non-renal increases in serum urea nitrogen include haemorrhage into the gastrointestinal tract with digestion and absorption of amino acids, fever, burns, corticosteroid administration and starvation/cachexia (Finco, 2008). Urea nitrogen is freely filtered across the glomerulus, and as much as 60% is reabsorbed in the renal tubules (Vander, 1980). The rate of tubular reabsorption is dependent upon the rate of tubular fluid flow: the higher the flow rate, the less reabsorption of urea. While clinically accessible, serum urea nitrogen is a measure of renal function only after 75% of the glomerular filtration rate has been diminished and is affected by non-renal factors, making it a relatively insensitive indicator of renal dysfunction.
Symmetric dimethylarginine
Symmetric dimethylarginine is a stable and continually released by-product of cellular protein methylation. The kidneys are the major route of elimination of symmetric dimethylarginine, and it is freely filtered by the glomerulus, with no reabsorption or secretion in the renal tubules (Hokamp and Nabity, 2016). Symmetric dimethylarginine is minimally affected by breed (Schott et al, 2021), age in adults (Schott et al, 2021; Siwinska et al, 2021), sex (Schott et al, 2021; Siwinska et al, 2021; Gough and McGovern, 2022) and size (Schott et al, 2021; Siwinska et al, 2021). Normal foals have significantly higher serum symmetric dimethylarginine concentrations than adults, which decrease rapidly within the first month of life, but remain elevated above the adult reference interval at 2–6 months of age (Bozorgmanesh et al, 2021; Siwinska et al, 2021; Gough and McGovern, 2022). Because age-appropriate reference intervals have not been established in foals, caution should be used interpreting symmetric dimethylarginine results in foals.
Serum symmetric dimethylarginine concentrations have been used in small animals as an early marker of decreased glomerular filtration rate in cases of chronic kidney disease, with increases seen earlier than creatinine, corresponding to a 40% reduction in glomerular filtration rate (Hall et al, 2014; 2016; Hokamp and Nabity, 2016; Relford et al, 2016). Several studies have evaluated the use of symmetric dimethylarginine in horses, with results revealing varying utility as a marker of glomerular filtration rate (Ertelt, 2021; Lo et al, 2021; Schott et al, 2021; Siwinska et al, 2021). Significant differences in symmetric dimethylarginine have been identified between healthy horses and horses with acute kidney injury (Siwinska et al, 2021; Lo et al, 2021) and horses with increasing levels of dehydration (Ertelt, 2021; Lo et al, 2021). One study found no difference in symmetric dimethylarginine between healthy horses and horses at risk of developing acute kidney injury, suggesting that symmetric dimethylarginine might not be useful as a biomarker of early decreased glomerular filtration rate in horses (Siwinska et al, 2021). However, cases reported in two other studies had increased symmetric dimethylarginine concentrations with normal creatinine, potentially indicative of early detection of decreased glomerular filtration rate (Schott et al, 2021; Lo et al, 2021).
Urine specific gravity
Another frequently used and simple measurement of renal function is urine specific gravity. Urine specific gravity may be used to categorise urine concentration as (Schott et al, 2018):
A urine specific gravity outside of the isosthenuric range reflects the ability of the kidneys to actively concentrate or dilute the urine. Animals with chronic kidney disease lose the ability to concentrate or dilute their urine when at least two-thirds of the nephrons become dysfunctional, and thus commonly have isosthenuric urine.
Fractional excretion of electrolytes
Fractional excretion of electrolytes in the urine can be used as estimates of renal tubular resorptive and secretory capacities. Fractional excretion is defined as the ratio of clearance of an electrolyte to the clearance of creatinine, and can be calculated as:
This calculation only requires simultaneous measurements of plasma and urine concentrations of the substance and creatinine, and thus is clinically feasible. Fractional excretion of sodium, potassium, chloride and phosphorus are used most commonly to assess renal function in horses (Schott et al, 2018). The normal equine kidney conserves more than 99% of all filtered sodium and chloride through reabsorption in the renal tubules, and normal fractional excretion of sodium and chloride is less than 1% (Schott et al, 2018). Increases in fractional excretion of sodium or chloride may represent tubular dysfunction through the inability to reabsorb these electrolytes. Fractional excretion should be interpreted in light of the animal's hydration status, fluid therapy, medication history or recent exercise, as all of these may significantly vary the fractional excretion of sodium and chloride.
Direct glomerular filtration rate measurement
While all of the previously discussed diagnostics are convenient to clinical practice, they can all be affected by non-renal factors, and are not sensitive to early or small changes in renal function. Quantitative measures of glomerular filtration rate can be categorised as plasma disappearance curves or clearance studies involving timed urine collections. The two techniques involve measuring an endogenous or exogenous substance and either its disappearance from the plasma and/or appearance in the urine. Methods involving the appearance of a substance in the urine usually involve collection of the total amount of urine collected during a 24-hour period, so are time-consuming and clinically impractical. Plasma disappearance studies involve measuring the disappearance of an exogenous marker from plasma.
Multiple plasma disappearance techniques for measuring glomerular filtration rate have been evaluated in horses. The traditional standard for measuring glomerular filtration is inulin clearance, but neither inulin nor its assay are commercially available, limiting the use of this method. Other markers have been used to estimate glomerular filtration rate, including radio-labelled pharmaceuticals such as 125I-iodothalamate and 99mTc-diethylenepentaacetic acid, and showed good correlation with inulin clearance (Matthews et al, 1992). However, these methods require specialised and careful handling of the compounds and are expensive to perform, limiting their use to facilities with the necessary equipment in clinical practice. Iohexol clearance has been used to estimate glomerular filtration rate in foals (Gonda et al, 2003), adult horses (Wilson et al, 2009; Meucci et al, 2015) and donkeys (Meucci et al, 2015). A simplified sample collection protocol, using clearance calculated from only three timepoints after iohexol administration, has been shown to correlate well with other methods of glomerular filtration rate measurement (Meucci et al, 2015). Iohexol clearance has been shown to be a safe, reliable and convenient method of estimation of glomerular filtration rate in horses, although the convenience depends upon the availability of iohexol and its assay.
Conclusions
The kidneys have many functions that are essential to homeostasis and life. Renal dysfunction is a commonly encountered clinical problem and can be detected through multiple accessible clinicopathologic tests. Commonly used tests, such as serum urea nitrogen, creatinine and urine specific gravity do not reflect early or small changes in renal function. Symmetric dimethylarginine has been used in horses, but more investigation into its accuracy in horses is necessary. The most accurate determination of renal function is measurement of glomerular filtration rate, but this requires specialised pharmaceuticals or assays, and can be time consuming. Interpretation of determinants of renal function in horses should be performed in light of their respective limitations.


